D05
Molecular principles of ER-phagy pathways
The endoplasmic reticulum (ER) is dynamically remodeled to adapt its structure to cellular needs. This process is mediated via selective autophagy (ER-phagy) involving reticulon-type receptors, namely FAM134B and RTN3. Here, we propose to analyze how the ER-phagy receptor FAM134B is regulated and how cargo selection is driven. In particular, we will investigate the interactome of FAM134B to identify potential regulators of ER-phagy. In addition, we aim to further study the role of ER chaperones Calnexin (CANX) and SIGMA-R1 that may act as FAM134B co-receptors for ER-phagy. Taken together, this study will help deciphering the role of ER-phagy in maintaining ER size, functionality, and turnover.
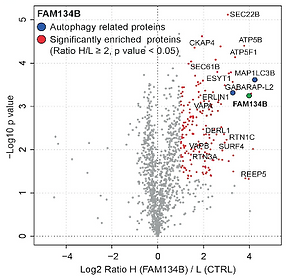
Volcano-plot for FAM134B SILAC-based interactome. To delineate the full interactomes of FAM134B, cells expressing FAM134B proteins and control cells were SILAC-labeled and treated with doxycycline for 24h and bafilomycin A1 for 4h. Peptides with Log2 Ratio H/L > 1 and –Log10 p value >1.3 are labeled in red. Data represent three biological replicates (Grumati et al., 2017).
Principal Investigators
Prof. Dr. Ivan dikic
Institute for Biochemistry II
GU Frankfurt
Theodor-Stern-Kai 7
60590 Frankfurt a. M.
Germany
Office: +49 69 6301-5652
dikic@biochem2.uni-frankfurt.de
Principal Investigators
Prof. Dr. GERHARD HUMMER
Max Planck Institute of Biophysics
Department of Theoretical Biophysics
Max-von-Laue-Str. 3
60438 Frankfurt am Main
Germany
Office: +49 69 6303-2501
office-hummer@biophys.mpg.de
